- Review
- Open access
- Published:
Dicoumarol: from chemistry to antitumor benefits
Chinese Medicine volume 17, Article number: 145 (2022)
Abstract
Dicoumarol, a coumarin-like compound, is known for its anticoagulant properties associated with the ability to inhibit vitamin K, being prescribed as a drug for several decades. The pharmaceutical value of dicoumarol turned it into a focus of chemists’ attention, aiming its synthesis and of dicoumarol derivatives, bringing to light new methodologies. In recent years, several other bioactive effects have been claimed for dicoumarol and its derivatives, including anti-inflammatory, antimicrobial, antifungal, and anticancer, although the mechanisms of action underlying them are mostly not disclosed and additional research is needed to unravel them. This review presents a state of the art on the chemistry of dicoumarols, and their potential anticancer characteristics, highlighting the mechanisms of action elucidated so far. In parallel, we draw attention to the lack of in vivo studies and clinical trials to assess the safety and efficacy as drugs for later application.
Introduction
Coumarins are a large family of compounds of natural or synthetic origin. In nature, coumarins are found in plants and microorganisms [1, 2]. Their structural variety and remarkable pharmacological activities, like antioxidant [3, 4], anti-inflammatory [5, 6], anticancer [7, 8], cardioprotective [9, 10], antimicrobial [11], antibacterial [12, 13], and enzymatic inhibition properties [14,15,16,17,18] are responsible for their top position in the field of natural products and medicinal chemistry [19, 20].
Dicoumarol, also known as bis-hydroxycoumarin, represents a good example of the benefits from the interaction of Western and traditional medicines. It was extracted and isolated for the first time in 1940 by Karl Link, from Melilotus officinalis (L.) Pall fungi [21]. This plant, commonly known as “yellow melilot” or “medicinal sweet clover,” is a species of the Fabaceae family and is widely distributed from Asia to Europe [22] and used as a medicine for centuries. Ancient Egyptians consumed tea made from this plant to treat earaches and intestinal worms. Also, this has been employed as a traditional Chinese herb with purifying and detoxifying properties [21]. In general, the beneficial blood-related qualities of M. officinalis, including lowering inflammation and increasing blood flow [23], are closely associated with coumarins, as they represent major compounds of this species. In this context, the isolation and recognition of the anticoagulant properties of dicoumarol led to its synthesis and of other derivatives having the same basic chemical structure.
Dicoumarol is well recognized for its anticoagulant and anti-inflammatory effects, and these properties have been reviewed by other authors [21, 24]. For the last years, this has also been highlighted to exert other important bioactive effects [21, 24]. To further understand these, it is crucial to discuss the chemistry of the compound in relation to its biological properties. Additionally, the compound’s anticancer effects are only briefly discussed, being important to distinguish the different mechanisms involved. Thus, this review summarizes the current level of knowledge about the chemistry of dicoumarols, their potential anticancer properties and the currently established mechanisms of action, for future medical applications.
General characterization of dicoumarol
Structure of dicoumarol
Chemically, dicoumarol 1, a 3,3′-methylenebis-(4-hydroxycoumarin) (1a,b, R=R′=H), consists of two cyclic β-ketoesters linked by a methylenic bridge (Fig. 1). The parent compound is 4-hydroxycoumarin (2, R=H), which can be represented as one of three tautomeric structures 2, 3 or 4 (Fig. 1). Several studies have demonstrated that coumarin form 2 is the main tautomer both in the solid state and in solution in polar solvents [25, 26]. Likewise, dicoumarols are often discussed because of their special molecular structures that may contain two intramolecular O–H\(\cdots\)O hydrogen bonds (1b) and distinct biological properties depending on the type of substituents on the central methylene linkage. A possible relationship between such hydrogen-bonded structure and the antimicrobial and antioxidant activities of dicoumarols is frequently suggested by different authors [27]. Still, for most publications cited in this review the structure shown for the synthesized dicoumarol derivatives is the one that corresponds to structure 1a.
Synthesis of dicoumarol
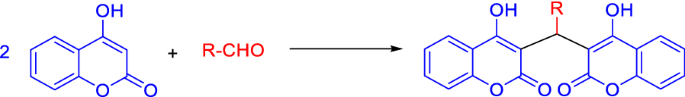
Due to the relevance of dicoumarol in the pharmaceutical field, the synthesis of this compound and its derivatives has been of major interest to chemists. Although there are some reports on the biosynthesis of dicoumarols by microorganisms that can use o-coumaric acid as the carbon source [28], and about their synthetic preparation starting from salicylic acid and formaldehyde [29], dicoumarols are generally synthesized by reaction of 4-hydroxycoumarin with different aldehydes in a 2:1 ratio. The position 3 in 4-hydroxycoumarin ring is highly activated, because of the influence of electron-donating hydroxy group and electron-withdrawing effects of carbonyl oxygen atom at the second position. There is a conjugation of π electrons from the double bond and the lone electron pairs in the oxygen atom. These two factors make the position 3 of coumarin ring very reactive for Michael addition reactions. In fact, the most common method for dicoumarols’ synthesis is the domino Knoevenagel–Michael reaction of 4-hydroxycoumarin with aldehyde derivatives and there have been developed several protocols for this reaction, that will be discussed in “Catalyst-free synthesis and synthesis using homogeneous catalysts” and “Synthesis using biocatalysts” sections.
Catalyst-free synthesis and synthesis using homogeneous catalysts
There are some publications on the synthesis of dicoumarols in the presence of 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) as non-nucleophilic and strong tertiary amine base [30], in phosphoroxychloride in dry DMF [31], in refluxing ethanol and acetic acid [27, 32,33,34,35], under microwave irradiation in ethanol or solvent-free conditions [32], in the presence of diethylaluminum chloride (Et2AlCl) in dichloromethane [36], in the presence of catalytic amount of piperidine in ethanol [37] or other polar solvents like methanol, dimethylformamide or dimethyl sulfoxide at room temperature [38], and using triethylamine in methanol or sodium ethoxide in ethanol in the presence of cyanogen bromide [39]. It was also published some uncatalyzed one-pot synthesis of dicoumarols under conventional or microwave thermal solvent-free conditions, which is a simple, practical, and environmentally benign method to obtain dicoumarols in excellent yields [30, 40, 41] as well as the catalyst-free one-pot synthesis in water under ultrasounds irradiation at ambient temperature [42] or the “on solvent” reaction using minimal amount of boiling propanol without any catalyst [43]. In 2012, Mallik and coworkers developed an “on water” methodology for the synthesis of dicoumarols at 95 °C for 4–5 h. In their study, they observed a significant reduction of reaction time from 4–5 h to 0.5 h, if an electrolyte was added to the reaction. Therefore, several dicoumarol derivatives were synthesized in very good yields (83–96%) in aqueous NaCl (5 M) solution at 95 °C [44]. Aiming to overcome some drawbacks of previously cited methods such as the use of toxic reagents, solvents or catalysts, high temperature, long reaction time, and tedious workup procedures, other protocols have been developed for this reaction (Table 1). Among these protocols, several use Lewis acids, like molecular iodine [45], MnCl2 [46], Zn(Proline)2 [47] and InCl3 under microwave irradiation [48] or sulfated titania (TiO2/SO42−) that behaves as Lewis and Bronsted acid [49] as catalysts. Inorganic acid salts like tris(hydrogensulfato)boron [B(HSO4)3] [50], and salts of transition metals such as ruthenium(III) chloride hydrate (RuCl3·nH2O) [51] have also been employed. With the increasing use of ionic liquids in organic synthesis, some methods have been developed using [bmim][BF4] [52], SO3H-functionalized ionic liquids [53], Brønsted acidic ionic liquids such as the 3-methyl-1-(4-sulfonic acid)butylimidazolium hydrogen sulfate [MIM(CH2)4SO3H][HSO4] under solvent-free conditions [54], tetramethylguanidium acetate ([TMG][Ac]) [55], choline hydroxide [56], poly(4-vinylpyridine)-supported ionic liquid ([P4VPy-BuSO3H]Cl-X(AlCl3)) [57, 58], 1,4-diazabicyclo[2.2.2]octane (Dabco)-based ionic liquid catalysts ([Dabco-H][AcO]) [58] or N-methylpyrrolidonium zinc chloride (Hnmp/ZnCl3)-based Brønsted–Lewis acidic ionic liquids [59]. Heteropoly acids, namely phosphotungstic acid [60], and phase transfer catalysts or surfactants like tetrabutylammonium bromide (TBAB) [61] and sodium dodecyl sulfate (SDS) [62] have also been used. Chemically modified glycerols, obtained by treatment of glycerol with agents that can react with hydroxy groups, possess physicochemical properties different from the parent glycerol and have been used as catalysts. For example, propane-1,2,3-triyl tris(hydrogen sulfate) (PTTH), prepared by addition of chlorosulfonic acid to glycerol at 0 °C, was employed in the synthesis of dicoumarols in water or solvent-free conditions at 80 °C with success [63]. The highly solving action of this catalyst on the reactants promotes their interaction and reactivity, and the carbonyl carbon of the aldehyde is activated by the intermolecular hydrogen bonding with the hydroxy groups of PTTH. In addition, the formed intermediates are stabilized by several types of complexations and hydrogen bonding with the hydroxy groups of PTTH. Most of the methods presented in Table 1 are of simple execution and are efficient since for most of them dicoumarol derivatives are obtained with very high yield and the reaction scope is broad because a wide range of aliphatic, and diversely substituted aromatic and heteroaromatic aldehydes, containing both electron-donating and electron-withdrawing substituents, are well-tolerated. Some of these methods are also considered environmentally friendly, especially those that use water as solvent or solvent-free neat conditions, thus avoiding the use of volatile and toxic organic solvents. In most of these methods [47, 49, 51, 52, 54,55,56,57,58,59,60, 62, 63] the catalyst can be recovered and reused, although with progressive loss of catalytic activity and consequent gradually decrease of reaction yields.
Regarding the reaction mechanism, this is similar for most of the methods reported in Table 1. The first step consists in the activation of the aldehyde through the binding of the catalyst (CAT) with the carbonyl oxygen to increase the electrophilicity of the carbonyl carbon of the aldehyde. Then a nucleophilic addition of 4-hydroxycoumarin to the catalyst–aldehyde complex (I) followed by elimination of water gives intermediate (II) which is further activated by the binding of the catalyst to the carbonyl oxygen (III) thus facilitating the reaction with another molecule of 4-hydroxycoumarin. Finally, an enolization occurs with concomitant formation of the dicoumarol (Fig. 2).
Synthesis using heterogeneous catalysts
As a result of the ongoing research for simpler, greener, and more efficient methods for the synthesis of dicoumarols, novel methodologies have been developed taking advantage of the introduction of more advanced tools in organic synthesis. In this sense, heterogeneous catalysts, including nanoparticles, and magnetic nanoparticles have gained much importance in recent years due to economic and environmental considerations. These catalysts are generally less expensive, highly reactive, eco-friendly, easy to handle, recover and reuse.
Davoodnia reported a highly efficient and fast method for the synthesis of dicoumarols, in ethanol, using tetrabutylammonium hexatungstate [TBA]2[W6O19] as a green and reusable heterogeneous catalyst (Scheme 1) [64]. For other solvents like methanol, chloroform, or acetonitrile, and in solvent-free conditions only moderate yields were achieved. Since the catalyst is not soluble in hot ethanol it can be easily recovered.
Another methodology was described for the synthesis of dicoumarol derivatives in water utilizing a polystyrene functionalized zinc anthranilic acid complex (PS-Zn-anthra complex) as a non-toxic and reusable Lewis acid catalyst (Scheme 2) [65]. The main advantages of this methodology are operational simplicity, mild reaction conditions, almost quantitative yields, short reaction time, low loading of catalyst, and the possibility to recycle the catalyst for five times without appreciable loss of its activity.
In 2010, Heravi et al. reported the synthesis of dicoumarols by one-pot domino Knoevenagel-type condensation/Michael reaction between 4-hydroxycoumarin and aromatic aldehydes in the presence of Preyssler type H14[NaP5W30O110] immobilized into SiO2 nanoparticles. By comparison with other catalysts and with the non-supported Preyssler type catalyst, the authors have shown that only 0.3 mol% of (H14[NaP5W30O110])/SiO2 can efficiently catalyze the reaction in ethanol and its reusability is another advantage (Scheme 3) [66]. Two years later, Niknam and coworkers published the use of silica-bonded N-propylpiperazine sodium n-propionate (SBPPSP) as a recyclable basic catalyst for the synthesis of dicoumarols in aqueous ethanol (1:1 v/v) under reflux conditions (Scheme 3) [67]. In 2013, another method was reported using silica sulfuric acid nanoparticles (SiO2–OSO3H NPs) as a solid acid catalyst under reflux in ethanol (Scheme 3) [68]. In the same year, Karimian et al. described the use of nano silica chloride (nano SiO2Cl) as an efficient, chemoselective and recyclable catalyst for facile and simple condensation of 4-hydroxycoumarin with aromatic and heteroaromatic aldehydes into dicoumarols in dry dichloromethane (Scheme 3) [69]. In turn, Ziarini et al. reported the synthesis of dicoumarols in the presence of sulfonic acid functionalized mesoporous silica SBA-15 as an efficient nanoporous heterogeneous solid acid catalyst using a mixture of H2O/EtOH (1:1) as solvent (Scheme 3) [70]. Later, other protocols for dicoumarols’ synthesis were developed using the silica-supported sodium hydrogen sulfate (NaHSO4·SiO2) and Indion 190 resin (Scheme 3) [71]. First, a methylidene-2H-chromene-2,4(3H)-dione intermediate is formed by the nucleophilic addition of 4-hydroxycoumarin to the activated aldehydes in the presence of NaHSO4·SiO2 or Indion 190 resin. Then, Michael addition of methylidene-2H-chromene-2,4(3H)-dione with a second unit of 4-hydroxycoumarin followed by dehydration afforded the expected dicoumarol [71]. The authors investigated the reusability of the catalyst. After completion of the reaction the catalyst was separated by filtration, washed with hexane, and dried. The activated catalyst was used for two more subsequent cycles with no loss of activity [71]. Since the discovery of MCM-41 (Mobil Composition of Matter No. 41) by Mobil Corporation scientists [72], these silica-based materials have been employed as efficient catalysts and catalyst supports. MCM-41 possesses several unique properties, including high surface area, uniform and tunable pore sizes, excellent physicochemical stability, and modifiable surfaces. However, its direct use as a catalyst is difficult due to a lack of sufficient acidity [73]. However, the functionalization of MCM-41 with transition metals, especially by manganese (Mn), leads to effective catalysts. One example, that has been used in the synthesis of dicoumarols is the solid acid catalyst Mn(pbyo)2Cl2/MCM-41 containing the 2,2′-bipyridine-6,6′-dionate (Scheme 3) [73].
Among the various nano catalysts magnesium oxide (MgO) finds out extensive application as heterogeneous catalyst. In 2014, Safaei-Ghomi and coworkers described magnesium oxide nanoparticles as an efficient, available, and cheap heterogeneous catalyst for the synthesis of dicoumarols in solvent free conditions (Scheme 4) [74]. Poly(vinylpyridine) (PVP) is an appropriate polymer for immobilization of nanoparticles because of the strong affinity of the pyridyl group to metals and its ability to make hydrogen bonds with polar species. In addition, PVP can interact electrostatically in quaternized or protonated forms with charged surfaces and therefore a variety of different PVP-supported reagents have been designed. In 2016, Shirini and coworkers reported the use of P4VPy–CuO-NPs as an effective catalyst for the synthesis of dicoumarols (Scheme 4) [75]. For the same purpose, 1 year later, bismuth vanadate nanoparticles (BiVO4-NPs) have also been used as an efficient and reusable nano-catalyst (Scheme 4). In the presence of this catalyst, yields of dicoumarols (95–98%) were superior compared to BiVO4 alone (75–95%) for a shorter reaction time [76].
Another example is the use of iron oxide nanoparticles (Fe3O4 NPs) as magnetically recyclable and safe catalyst for the green synthesis of dicoumarols via the one-pot condensation of 4-hydroxycoumarin with aryl glyoxals on water (Scheme 5) [77]. Catalyst loadings can be as low as 2 mol% to give high product yield. Other advantages of this method are the avoidance of toxic organic solvents, and simple work-up procedure.
Mechanistically, in the presence of Fe3O4 NPs an active leaving group produced on aryl glyoxal by a Lewis acid such as iron(II, III) may be a reasonable start for the Knoevenagel condensation with 4-hydroxycoumarin (2). Subsequently, 1,4-addition of next 4-hydroxycoumarin (2) to the formed α,β-unsaturated ketone gave the target molecule. This mechanism also shows the recyclability of Fe3O4 NPs (Fig. 3) [77].
The combination of water as a solvent with the use of immobilized catalysts that can be recovered and reused is even more desirable. A green synthesis of dicoumarol through reaction of 4-hydroxycoumarin with various aldehydes was achieved, in excellent yields and high rates, by using Ni-dimethylglyoxime complex immobilized on functionalized Fe3O4 by a post-grafting process (Fe3O4@SiO2-SP-DMG-Ni(II) NMPs) as a superparamagnetic catalyst (Scheme 6) [78]. This catalyst proved to be thermally stable, and efficient for this synthesis and was conveniently recovered using an external magnetic field and reused for subsequent reactions at least seven times without a significant decrease in catalytic activity.
Regarding the reaction mechanism, the Fe3O4@SiO2-SP-DMG-Ni(II) NMPs behave as a Lewis acid catalyst and activate the carbonyl group of the aldehyde, allowing immediate Knoevenagel condensation between 4-hydroxycoumarin and benzaldehyde. Then, water elimination gives Michael acceptor intermediate that undergoes a Michael addition with another 4-hydroxycoumarin and after enolization, the dicoumarol is obtained [78].
Some recent works have described the synthesis of dicoumarols catalyzed by organocatalysts. For example, the use of trityl bromide (TrBr or Ph3CBr) as a neutral organocatalyst for the synthesis of dicoumarols in mild and solvent-free conditions was reported and the performance of this catalyst was found to be comparable to that of the nano-magnetic catalyst [Fe3O4@SiO2@(CH2)3-Im-SO3H]Cl (Scheme 7) [78].
A work published in 2015, has demonstrated that the encapsulation of l-tyrosine organocatalyst into nanoparticles increases its ability to catalyze organic reactions [79]. In fact, l-tyrosine loaded nanoparticles (LTNPs) were used as catalyst in the synthesis of dicoumarol derivatives which were obtained in good yields (85–93%) in a short period of time (5–15 min) (Scheme 8). It was observed that with increment in the dielectric constant of protic solvents the catalyst gives higher yield in lesser time, which culminated in the choice of water as solvent. Increment in the catalyst surface area, very small catalyst loading, and easy separation of the catalyst are key advantages of this method.
Synthesis using biocatalysts
The use of biocatalysts in the Knoevenagel–Michael reaction for dicoumarol synthesis has been much less explored. Yet, a recent work was published using this type of catalysts. Hu and coworkers reported the use of the enzyme lipase from fungus Rhizomucor miehei (lipase RMIM) as a biocatalyst in the Knoevenagel–Michael cascade reaction of 4-hydroxycoumarin with aromatic, heterocyclic or aliphatic aldehydes to synthesize dicoumarol derivatives in water with excellent yields (81–98%) (Scheme 9) [80]. The mild reaction conditions, pure aqueous reaction system, broad substrate scope, recyclability of the catalyst, and operational simplicity make this an environmentally friendly process for dicoumarols’ synthesis. The first step of the reaction mechanism involves the interaction of the His-Asp residue of lipase with the hydroxyl group of 4-hydroxycoumarin to activate the compound for the Knoevenagel condensation with the aldehyde moiety, and the following steps involve a Mickael addition and enolization step as already described for the dicoumarols synthesis in other reaction conditions [80].
Current and future potential medical applications
Dicoumarol is well-known for its pharmacological properties, mainly anticoagulant and anti-inflammatory activities. In fact, this was the first of the oral anticoagulants to be isolated and employed clinically. A vitamin K-dependent step in the hepatic synthesis of clotting factors II (prothrombin), VII, IX, and X is inhibited, leading to its anticoagulant activity. In particular, dicoumarol suppresses the carboxylation of glutamate residues in the N-terminal sections of vitamin K-dependent proteins, by impeding vitamin K reductase. This event limits the activation of vitamin K-dependent clotting factors, resulting in a decrease in prothrombin levels and consequently a decrease in clotting [81]. In this way, it acts as a vitamin K antagonist by inhibiting its bioavailability. It should be noted that dicoumarol acts to prevent the development of new thrombi but not to eliminate existing thrombi. This substance’s slow and irregular absorption can lead to gastrointestinal issues, main reasons why it has largely been replaced by warfarin [82].
Dicoumarol and associated anticoagulants are hydroxylated at the 4 position, which is necessary for potent anticoagulant action among other factors. Although several of the common plant coumarins do have very modest action when fed to animals in high doses, none of them have considerable clinical anticoagulant activity because they are not all replaced at this location [83]. Bleeding is the predominant adverse reaction of dicoumarol therapy. Hepatitis, hypersensitivity, and cholesterol embolization are a few more side effects that might manifest. Dicoumarol may be contraindicated alone or in combination with other thrombolytic drugs and clotting inhibitors [82]. Additionally, the European Medicined Agency recommended using dicoumarol for earaches and eye ulcers [22]. Currently, in the Netherlands, Poland, Austria, and Germany it is used as a medicine with antioxidant and anticoagulant activities, in the United Kingdom it is used to treat edema and for the circulation of the renal vein [84].
In addition to the well-recognized pharmacological properties of dicoumarol, it is known to have other activities of interest for future applications, including as anticancer (detailed in “Antitumor action of dicoumarol” section) and as antimicrobial agents. Dicoumarol was initially discovered as a rodenticide [85] and that said, several in vitro studies have explored its potential as an antimicrobial agent, namely antibacterial, antiviral, and anti-fungal. Dholariya et al. [86] conducted a study where they analyzed the antibacterial activity of dicoumarol and a series of its derivatives containing Cu2+ complexes. The antibacterial activity was exerted for the gram-negative bacterium (Pseudomonas aeruginosa ATCC25619 and Escherichia coli ATCC25922) and gram-positive bacterium (Bacillus subtilis ATCC11774 and Streptococcus pyogenes ATCC12384). Moreover, it also exerted antifungal activity against Aspergillus niger ATCC64958 and Candida albicans ATCC6602. Dicoumarol also exerted anti-tubercular activity for Mycobacterium tuberculosis (H37Rv and H37Ra strains) [86, 87], further demonstrating that this compound increased the sensitivity of anti-tuberculosis drugs (such as isoniazid and rifampicin) when it was present at a low concentration [87]. Dicoumarol inhibits human immunodeficiency virus-1 (HIV-1) gene replication by degrading Tat (a protein encoded by HIV-1) through inhibition of NAD(P)H:quinone oxidoreductase, allowing steady-state levels to maintain capacity in viral transcripts [88]. In the study performed by Kammari et al. [89], the authors validated its anti-HIV activity. They observed anti-HIV-1 activity using dicoumarol pyridine derivatives with potential activity against a topoisomerase IIβ kinase, which is present in HIV-1 viral lysate. In another study, four synthetics derivatives dicoumarols (DC, 2-PyDC, 3-PyDC and 4-PyDC) were synthesized and characterized. All compounds showed inhibition in four Staphylococcus aureus bacterial strains such as S. aureus ATCC 29213, methicillin-resistant S. aureus (MRSA XJ 75302), vancomycin-intermediate S. aureus (Mu50 ATCC 700699), and USA 300 (Los Angeles County clone, LAC). Antibacterial activity was demonstrated for a minimum inhibitory concentration between 16 and 64 μg/mL, with the dicoumarol derivative, 2-PyDC (2-Pyridinodicoumarol), showing the most potent antibacterial activity [90].
Antitumor action of dicoumarol
Dicoumarol has been gaining interest in the cancer setting due to its action as an inhibitor of nicotinamide adenine dinucleotide phosphate (NAD(P)):(quinone acceptor) oxidoreductase 1 (NQO1), by competing with NAD(P)H at the pyridine nucleotide binding site [91]. NQO1 is a cytosolic flavoenzyme that catalyzes the two-electron reduction of several quinone substrates to give their corresponding hydroquinones using both NADH and NADPH as electron donors [92], therefore avoiding the generation of reactive oxygen species (ROS) by redox cycling and functioning as an effective superoxide scavenger [92]. The toxicity of dicoumarol against cancer cells seems to be mainly mediated by the mitochondrial production of ROS due to NQO1 inhibition (Table 2) [93]. Nonetheless, other mechanisms independent of NQO1 inhibition have also been uncovered.
Since the pharmacological and biological effects of dicoumarol have been explored in several contexts, its derivatives have also been gaining interest by the scientific community; however, the information available about the antitumor action of such derivatives is scarce [94, 95]. For instance, a study showed that the dicoumarol derivative spindlactone A (SPL-A) sensitized endometrial cancer Ishikawa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through the downregulation of the expression of cellular FLICE-like inhibitory protein (c-FLIP), Bcl-2, Bcl-xl and Mcl-1 and the upregulation of p53 expression, cleaved poly-(adenosine diphosphate-ribose) polymerase (PARP) levels, and caspase activity [96]. The apoptotic process in these cells was probably triggered by an SPL-A-induced increase in intracellular ROS levels via the inhibition of NQO1 [96]. It would be important to unravel derivatives with clinical potential that do not exhibit the off-target effects of dicoumarol (e.g., increasing oxygen radical formation and inhibition of oxygen respiration in normal cells) [94].
NQO1 inhibition-dependent actions
By inhibiting NQO1, dicoumarol triggered oxidative stress (given by the increased intracellular production of superoxide anion and glutathione disulfide (GSSG)) and apoptosis and also inhibited cell growth of human MIA PaCa-2 pancreatic cancer cells [97, 104]. Apoptosis of this cell line seemed to be mediated by a dicoumarol-induced increase in cytosolic cytochrome c and cleaved PARP levels in a dose- and time-dependent manner [97]. Mechanistically, when the mitochondrial membrane permeability is disrupted, cytochrome c is released from mitochondria to the cytosol where it complexes with apoptotic protease-activating factor (Apaf) to activate caspase-9, which in turn can activate downstream caspases, like caspase-3. Caspase-3 is cleaved into its active form, which cleaves its substrate PARP, which is a marker of apoptosis [105]. These effects were confirmed in vivo with dicoumarol decreasing tumor growth in nude mice inoculated with MIA PaCa-2 cells and increasing the overall survival of the animals [97].
Data suggest that the inhibitory action of dicoumarol in NQO1 may also have a synergistic effect with other compounds via a mechanism based on p53 stabilization through NQO1. Dicoumarol, by inhibiting NQO1, induces p53 degradation, inhibiting p53-dependent p21 induction, and consequently, enhancing the apoptotic response [100]. This mechanism has been observed in several studies. Pretreatment of human colon cancer HCT116 cells with dicoumarol promoted miltirone-induced mitochondrial damage and apoptosis, effects that may be possibly the result of the dicoumarol-induced decrease of p53 stability due to NQO1 inhibition [98]. Dicoumarol also sensitized urothelial cancer cells with wild-type p53 (functional p53 seems to be necessary for the synergistic effect) to doxorubicin through p38 activation, which was induced by the suppression of the p53–p21 pathway [100]. Similarly, it enhanced the cytotoxicity of cisplatin in urogenital cancer cells with wild-type p53 through the suppression of p53–p21 pathway, which led possibly to JNK activation and apoptosis [99]. Moreover, this has been shown to enhance gemcitabine cytotoxicity in high NQO1 activity cholangiocarcinoma cells [106]. The authors suggested that the mechanism of dicoumarol-induced cell killing was not mediated by mitochondrial impairment and formation of ROS but related to the suppression of the pro-survival response to chemotherapy [106]. The combined therapy of dicoumarol with anticancer drugs may provide a novel therapeutic option to overcome chemoresistance in cancer treatment.
NQO1 inhibition-independent actions
Dicoumarol effects on cancer cells may also be independent of its inhibitory action on NQO1 and several mechanisms have been proposed: (i) impairment of mitochondrial functionality; (ii) decrease of pregnancy-specific beta-1-glycoprotein 1 (PSG1) levels; (iii) inhibition of heat shock protein 90 (Hsp90); (iv) downregulation of anti-apoptotic proteins; (v) inhibition of heat shock protein 90 (Hsp90); and (vi) inhibition of phosphoinositide-dependent kinase-1 (PDK1).
Dicoumarol was reported to inhibit mitochondrial oxidative phosphorylation (OXPHOS) complexes II, III and IV, to stimulate superoxide anion release by reversed electron flow at OXPHOS complex II, and to inhibit the biosynthesis of pyrimidine at the dihydroorotate dehydrogenase step, leading to the accumulation of S phase cells in human myeloid leukemia HL-60 cell line under conditions of complete depletion of NQO1 [91].
In estrogen receptor-negative breast cancer (in vitro and in vivo), dicoumarol counteracted the chemoresistance to taxane-anthracycline-based chemotherapy by targeting the PSG1 [101]. Specifically, it decreased PSG1 levels and extracellular secretion, which led to the decrease in the activation of the PSG1 downstream mediator transforming growth factor β (TGFβ)1 [101]. TGFβ1 is an important contributor to cancer progression, including chemoresistance, since it induces pro-proliferation signals, such as epithelial-mesenchymal transition (EMT) signal [101]. The expression of EMT hallmarks, namely N-cadherin, vimentin and fibronectin, were inhibited by dicoumarol [101].
It was also demonstrated that dicoumarol treatment sensitized TRAIL-mediated apoptosis since it downregulated Bcl-2 expression by inhibiting nuclear factor kappa B (NF-κB) and CRE transcriptional activities, and Mcl-1 and cellular FLICE-like inhibitory protein (c-FLIP) expressions at the post-translational level (possibly by affecting protein stability). TRAIL is a known inducer of apoptosis, an effect that seems to be specific for cancer cells; however, TRAIL resistance is observed in several cancers [102]. In this way, dicoumarol may be used as a potential sensitizer for the treatment of TRAIL-resistant renal cancer [102].
Dicoumarol also downregulated PTTG1/Securin expression (a cell cycle protein that is elevated in many tumor types) by inhibiting Hsp90 (whose activity is increased in all cancers) [107]. Hsp90 inhibition leads to the downregulation of signaling pathways involved in proliferation, such as phosphoinositide 3-kinase (PI3K)/AKT and Raf-1, and to the induction of cell death by activating intrinsic and extrinsic pathways of apoptosis [107].
Another mechanism triggered by dicoumarol, independent of NQO1, seems to be the inhibition of PDK1, a key enzyme that negatively regulates the activity of the pyruvate dehydrogenase (PDH) complex by phosphorylation [103]. Inactivation of the PDH is associated with the Warburg effect (cancer cells rely on aerobic glycolysis—conversion of glucose to lactate even in the presence of oxygen—for energy production) [103, 108]. Dicoumarol inhibited the kinase activity of PDK1 in human ovarian cancer cells, shifting the glucose metabolism from aerobic glycolysis to OXPHOS (given the elevated glucose uptake and decreased lactate production), generating higher levels of ROS. Therefore, the mitochondrial membrane potential (MMP) was attenuated, inducing apoptosis, and reducing cell viability [103]. In vivo, dicoumarol was able to reduce tumor growth, possibly by targeting the kinase activity of PDK1, which induced the apoptosis of tumor cells [103]. Mechanistically, PDK1 inhibition results in an elevated influx of acetyl-CoA into the Krebs cycle, promotes NADH delivery to OXPHOS complex I, and induces an elevated production of ROS, which in turn damages redox-sensitive complex I and the proton pump [103]. Consequently, protons are incapable of efflux through the mitochondrial inner membrane, resulting in a decreased MMP (cancer cells exhibit a hyperpolarized MMP compared with normal cells) [103]. MMP depolarization opens the mitochondrial transition pore, resulting in the efflux of cytochrome c that makes part of the apoptosome. The pro-apoptotic proteins caspase-3 and PARP are then cleaved, leading to the activation of apoptosis, and therefore, to the suppression of tumor growth and reduction of cell viability both in vitro and in vivo [103]. Figure 4 overviews the main pathways regulated by dicoumarol in tumor cells.
Actions of dicoumarol that may contribute to its antitumor effects. The figure was produced using Servier Medical Art. Legend—I, II, III, IV: oxidative phosphorylation complexes I, II, III and IV, respectively; cyt c: cytochrome c; CRE: cyclic adenose monophosphate response elements; JNK: c-Jun N-terminal kinase; NADH: nicotinamide adenine dinucleotide; NF-κB: nuclear factor kappa B; NQO1: NAD(P):(quinone acceptor) oxidoreductase 1; PARP: poly (adenosine diphosphate-ribose) polymerase; PDH: pyruvate dehydrogenase; PDK1: phosphoinositide-dependent kinase-1; PI3K: phosphoinositide 3-kinase; PSG1: pregnancy specific beta-1-glycoprotein 1; ROS: reactive oxygen species; TRAIL: Tumor necrosis factor-related apoptosis-inducing ligand; TGFβ1: transforming growth factor beta 1; TFGβR: transforming growth factor receptor
Hitherto the majority of the data available on the effects of dicoumarol in cancer treatment are derived from in vitro studies. Therefore, preclinical studies are needed to validate data from in vitro studies and assess the putative therapeutic effects of dicoumarol, particularly, in targeting tumor growth and chemoresistance.
Conclusions
The discovery of dicoumarol, a symmetrical natural biscoumarin, with important pharmacological activities, instigated numerous studies towards the development of efficient methods for its synthesis and of related derivatives, and investigation of their bioactivities. Among the synthetic methods, the most promising are those involving heterogeneous catalysis and the use of green solvents, which present economic advantages due to the possibility of recovering and reusing the catalyst and are also more sustainable.
In addition to its anticoagulant properties, dicoumarol has also gained a lot of interest due to other bioactive properties. Its anticancer properties are commonly associated to its ability to inhibit NQO1, but other effects, such as (i) impairment of mitochondrial functionality; (ii) decrease of PSG1 levels; (iii) inhibition of Hsp90; (iv) downregulation of anti-apoptotic proteins; and (v) inhibition of PDK1 may also occur. In recent years, a variety of cancer types, including colon, breast, ovarian, kidney, lung, urothelial, prostate, glioblastoma, and myeloid leukemia, have been shown to respond favorably to dicoumarol. However, although there is some in vitro evidence, further in vivo experiments and clinical trials are essential to establish the efficacy and safety of the mechanisms of action of dicoumarol and its derivatives. This review, devoted to dicoumarol and its derivatives, encloses the most relevant data related to their synthesis and mechanisms of antitumoral activity, which are crucial factors to reveal having in mind their future medicinal applications. Despite these stated evidences, further studies should be carried out to better understand the potential of dicoumarol derivatives in anti-cancer mechanisms, due to the lack of research in this area.
Availability of data and materials
Not applicable
References
Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity. Curr Med Chem. 2005;12:887–916. https://doi.org/10.2174/0929867053507315.
Hussain MI, Syed QA, Khattak MNK, Hafez B, Reigosa MJ, El-Keblawy A. Natural product coumarins: biological and pharmacological perspectives. Biologia. 2019;74:863–88. https://doi.org/10.2478/s11756-019-00242-x.
Al-Majedy Y, Al-Amiery A, Kadhum AA, BakarMohamad A. Antioxidant activity of coumarins. Syst Rev Pharm. 2016;8:24–30. https://doi.org/10.5530/srp.2017.1.6.
Kostova I, Bhatia S, Grigorov P, Balkansky S, Parmar VS, Prasad AK, Saso L. Coumarins as antioxidants. Curr Med Chem. 2011;18:3929–51. https://doi.org/10.2174/092986711803414395.
Grover J, Jachak SM. Coumarins as privileged scaffold for anti-inflammatory drug development. RSC Adv. 2015;5:38892–905. https://doi.org/10.1039/C5RA05643H.
Wei W, Wu X-W, Deng G-G, Yang X-W. Anti-inflammatory coumarins with short- and long-chain hydrophobic groups from roots of Angelica dahurica Cv. Hangbaizhi. Phytochemistry. 2016;123:58–68. https://doi.org/10.1016/j.phytochem.2016.01.006.
Thakur A, Singla R, Jaitak V. Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. Eur J Med Chem. 2015;101:476–95. https://doi.org/10.1016/j.ejmech.2015.07.010.
Bhattarai N, Kumbhar AA, Pokharel YR, Yadav PN. Anticancer potential of coumarin and its derivatives. Mini-Rev Med Chem. 2021;21:2996–3029. https://doi.org/10.2174/1389557521666210405160323.
Vilar S, Quezada E, Santana L, Uriarte E, Yánez M, Fraiz N, Alcaide C, Cano E, Orallo F. Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids. Bioorg Med Chem Lett. 2006;16:257–61. https://doi.org/10.1016/j.bmcl.2005.10.013.
Najmanova I, Dosedel M, Hrdina R, Anzenbacher P, Filipsky T, Riha M, Mladenka P. Cardiovascular effects of coumarins besides their antioxidant activity. Curr Top Med Chem. 2015;15:830–49. https://doi.org/10.2174/1568026615666150220112437.
Al-Majedy YK, Kadhum AAH, Al-Amiery AA, Mohamad AB. Coumarins: the antimicrobial agents. Syst Rev Pharm. 2017;8:62–70. https://doi.org/10.5530/srp.2017.1.11.
Joao Matos M, Vazquez-Rodriguez S, Santana L, Uriarte E, Fuentes-Edfuf C, Santos Y, Munoz-Crego A. Looking for new targets: simple coumarins as antibacterial agents. Med Chem. 2012;8:1140–5. https://doi.org/10.2174/1573406411208061140.
Yang L, Ding W, Xu Y, Wu D, Li S, Chen J, Guo B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016;21:468. https://doi.org/10.3390/molecules21040468.
Matos MJ, Viña D, Janeiro P, Borges F, Santana L, Uriarte E. New halogenated 3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2010;20:5157–60. https://doi.org/10.1016/j.bmcl.2010.07.013.
Matos MJ, Viña D, Picciau C, Orallo F, Santana L, Uriarte E. Synthesis and evaluation of 6-methyl-3-phenylcoumarins as potent and selective MAO-B inhibitors. Bioorg Med Chem Lett. 2009;19:5053–5. https://doi.org/10.1016/j.bmcl.2009.07.039.
Matos MJ, Terán C, Pérez-Castillo Y, Uriarte E, Santana L, Viña D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J Med Chem. 2011;54:7127–37. https://doi.org/10.1021/jm200716y.
Delogu G, Picciau C, Ferino G, Quezada E, Podda G, Uriarte E, Viña D. Synthesis, human monoamine oxidase inhibitory activity and molecular docking studies of 3-heteroarylcoumarin derivatives. Eur J Med Chem. 2011;46:1147–52. https://doi.org/10.1016/j.ejmech.2011.01.033.
Asghar H, Asghar H, Asghar T. A review on anti-urease potential of coumarins. Curr Drug Targets. 2021;22:1926–43. https://doi.org/10.2174/1389450122666210222091412.
Borges MFM, Roleira FMF, Milhazes NJSP, Villare EU. Simple coumarins: privileged scaffolds in medicinal chemistry. In: Frontiers in medicinal chemistry, vol. 4. Sharjah: Bentham Science Publishers; 2012. p. 23–85.
Garg SS, Gupta J, Sharma S, Sahu D. An insight into the therapeutic applications of coumarin compounds and their mechanisms of action. Eur J Pharm Sci. 2020;152: 105424. https://doi.org/10.1016/j.ejps.2020.105424.
Sun C, Zhao W, Wang X, Sun Y, Chen X. A pharmacological review of dicoumarol: an old natural anticoagulant agent. Pharmacol Res. 2020;160: 105193.
Ilhan M, Ali Z, Khan IA, KüpeliAkkol E. A new isoflavane-4-ol derivative from Melilotus officinalis (L.) Pall. Nat Prod Res. 2019;33:1856–61. https://doi.org/10.1080/14786419.2018.1477152.
Chorepsima S, Tentolouris K, Dimitroulis D, Tentolouris N. Melilotus: contribution to wound healing in the diabetic foot. J Herb Med. 2013;3:81–6.
Zaki Rashed KN. Biological evidences of dicoumarol: a review. Plantae Scientia. 2021;4:121–4. https://doi.org/10.32439/ps.v4i2.121-124.
Arndt F, Loewe L, Ün R, Ayça E. Cumarindiol und Cumarin-Chromon-Tautomerie. Chem Ber. 1951;84:319–29. https://doi.org/10.1002/cber.19510840312.
Farmer VC. Spectra and structure of 4-hydroxycoumarins. Spectrochim Acta. 1959;15:870–82. https://doi.org/10.1016/S0371-1951(59)80384-2.
Hamdi N, Puerta MC, Valerga P. Synthesis, structure, antimicrobial and antioxidant investigations of dicoumarol and related compounds. Eur J Med Chem. 2008;43:2541–8. https://doi.org/10.1016/j.ejmech.2008.03.038.
Bellis D, Spring M, Stoker J. The biosynthesis of dicoumarol. Biochem J. 1967;103:202–6. https://doi.org/10.1042/bj1030202.
Sanjeeva Reddy C, Raghu M. Synthesis of novel 6,6′-methylene-bis-[3-(2-anilinoacetyl)-4-hydroxycoumarin] derivatives. Chem Pharm Bull. 2008;56:1732–4. https://doi.org/10.1248/cpb.56.1732.
Hagiwara H, Fujimoto N, Suzuki T, Ando M. Synthesis of methylenebis(4-hydroxy-2-pyrone) or methylenebis(4-hydroxycoumarin) derivatives by organic solid state reaction. Heterocycles. 2000;53:549. https://doi.org/10.3987/COM-99-8817.
Elgamal MHA, Shalaby NMM, Shaban MA, Duddeck H, Mikhova B, Simon A, Toth G. Synthesis and spectroscopic investigation of some dimeric coumarin and furanocoumarin models. Monatshefte für Chemie/Chem Mon. 1997;128:701–12. https://doi.org/10.1007/BF00807602.
Qadir S, Dar AA, Khan KZ. Synthesis of biscoumarins from 4-hydroxycoumarin and aromatic aldehydes—a comparative assessment of percentage yield under thermal and microwave-assisted conditions. Synth Commun. 2008;38:3490–9. https://doi.org/10.1080/00397910802162942.
Zhao H, Neamati N, Hong H, Mazumder A, Wang S, Sunder S, Milne GWA, Pommier Y, Burke TR. Coumarin-based inhibitors of HIV integrase. J Med Chem. 1997;40:242–9. https://doi.org/10.1021/jm960450v.
Manolov I, Maichle-Moessmer C, Danchev N. Synthesis, structure, toxicological and pharmacological investigations of 4-hydroxycoumarin derivatives. Eur J Med Chem. 2006;41:882–90. https://doi.org/10.1016/j.ejmech.2006.03.007.
Jung J-C, Park O-S. Synthetic approaches and biological activities of 4-hydroxycoumarin derivatives. Molecules. 2009;14:4790–803. https://doi.org/10.3390/molecules14114790.
Hagiwara H, Miya S, Suzuki T, Ando M, Yamamoto I, Kato M. Et2AlCl promoted coupling reactions of 4-hydroxy-2-pyrone or 4-hydroxycoumarine with aldehydes: synthesis of methylenebis-(4-hydroxy-2-pyrone) or methylenebis-(4-hydroxycoumarine) derivatives. Heterocycles. 1999;51:493. https://doi.org/10.3987/COM-98-8429.
Khan KM, Iqbal S, Lodhi MA, Maharvi GM, Ullah Z, Choudhary MI, Rahman A, Perveen S. Biscoumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg Med Chem. 2004;12:1963–8. https://doi.org/10.1016/j.bmc.2004.01.010.
Kumar A, Gupta MK, Kumar M. An efficient non-ionic surfactant catalyzed multicomponent synthesis of novel benzylamino coumarin derivative via Mannich type reaction in aqueous media. Tetrahedron Lett. 2011;52:4521–5. https://doi.org/10.1016/j.tetlet.2011.06.040.
Imani M, NorooziPesyan N, Aalinejad M, Şahin E. Study of chemical behaviors of 4-hydroxycumarin in alkali media: dicumarols or dihydro-4H-furo[3,2-c]chromenes? J Iran Chem Soc. 2022;19:3397–405. https://doi.org/10.1007/s13738-022-02533-8.
Shaterian HR, Honarmand M. Uncatalyzed, one-pot synthesis of 3,3′-(benzylene)-bis(4-hydroxy-2H-chromen-2-one) derivatives under thermal solvent-free conditions. Chin J Chem. 2009;27:1795–800. https://doi.org/10.1002/cjoc.200990302.
Das Gupta A, Samanta S, Mondal R, Mallik AK. A rapid, efficient and green method for synthesis of 3,3′-arylmethylene-bis-4-hydroxycoumarins without use of any solvent, catalyst or solid surface. Chem Sci Trans. 2013;2:524–8. https://doi.org/10.7598/cst2013.388.
Al-Kadasi AMA, Nazeruddin GM. Ultrasound assisted catalyst-free one-pot synthesis of bis-coumarins in neat water. Int J Chem Sci. 2012;10:324–30.
Elinson M, Vereshchagin AN, Sokolova OO. Fast highly efficient “on-solvent” non catalytic cascade transformation of benzaldehydes and 4-hydroxycoumarin into bis(4-hydroxycoumarinyl)arylmethanes. ARKIVOC. 2017;2017:121–9. https://doi.org/10.24820/ark.5550190.p010.023.
Das Gupta A, Samanta S, Mondal R, Mallik AKA. Convenient, eco-friendly, and efficient method for synthesis of 3,3′-arylmethylene-bis-4-hydroxycoumarins “on-water.” Bull Korean Chem Soc. 2012;33:4239–42. https://doi.org/10.5012/bkcs.2012.33.12.4239.
Kidwai M, Bansal V, Mothsra P, Saxena S, Somvanshi RK, Dey S, Singh TP. Molecular iodine: a versatile catalyst for the synthesis of bis(4-hydroxycoumarin) methanes in water. J Mol Catal A Chem. 2007;268:76–81. https://doi.org/10.1016/j.molcata.2006.11.054.
Sangshetti JN, Kokare ND, Shinde DB. Water mediated efficient one-pot synthesis of bis-(4-hydroxycoumarin)methanes. Green Chem Lett Rev. 2009;2:233–5. https://doi.org/10.1080/17518250903393874.
Siddiqui ZN, Farooq F. Zn(Proline)2: a novel catalyst for the synthesis of dicoumarols. Catal Sci Technol. 2011;1:810. https://doi.org/10.1039/c1cy00110h.
Simijonović D, Vlachou E-E, Petrović ZD, Hadjipavlou-Litina DJ, Litinas ΚE, Stanković N, Mihović N, Mladenović MP. Dicoumarol derivatives: green synthesis and molecular modelling studies of their anti-LOX activity. Bioorg Chem. 2018;80:741–52. https://doi.org/10.1016/j.bioorg.2018.07.021.
Karmakar B, Nayak A, Banerji J. Sulfated titania catalyzed water mediated efficient synthesis of dicoumarols—a green approach. Tetrahedron Lett. 2012;53:4343–6. https://doi.org/10.1016/j.tetlet.2012.06.024.
Karimi-Jaberi Z, Nazarifar MR, Pooladian B. Tris(hydrogensulfato) boron as a solid heterogeneous catalyst for the rapid synthesis of α, Α′-benzylidene bis(4-hydroxycoumarin) derivatives. Chin Chem Lett. 2012;23:781–4. https://doi.org/10.1016/j.cclet.2012.05.003.
Tabatabaeian K, Heidari H, Khorshidi A, Mamaghani M, Mahmoodi N. Synthesis of biscoumarin derivatives by the reaction of aldehydes and 4-hydroxycoumarin using ruthenium (III) chloride hydrate as a versatile homogeneous catalyst. J Serb Chem Soc. 2012;77:407–13. https://doi.org/10.2298/JSC110427189T.
Khurana JM, Kumar S. Ionic liquid: an efficient and recyclable medium for the synthesis of octahydroquinazolinone and biscoumarin derivatives. Monatshefte für Chemie/Chem Mon. 2010;141:561–4. https://doi.org/10.1007/s00706-010-0306-4.
Li W, Wang Y, Wang Z, Dai L, Wang Y. Novel SO3H-functionalized ionic liquids based on benzimidazolium cation: efficient and recyclable catalysts for one-pot synthesis of biscoumarin derivatives. Catal Lett. 2011;141:1651–8. https://doi.org/10.1007/s10562-011-0689-9.
Tavakoli-Hoseini N, Heravi MM, Bamoharram FF, Davoodnia A, Ghassemzadeh M. An unexpected tetracyclic product isolated during the synthesis of biscoumarins catalyzed by [MIM(CH2)4SO3H][HSO4]: characterization and X-ray crystal structure of 7-(2-hydroxy-4-oxo-4H-chromen-3-yl)-6H,7H-chromeno[4,3-b]chromen-6-one. J Mol Liq. 2011;163:122–7. https://doi.org/10.1016/j.molliq.2011.08.007.
Zhu A, Wang M, Li L, Wang J. Tetramethylguanidium-based ionic liquids as efficient and reusable catalysts for the synthesis of biscoumarin at room temperature. RSC Adv. 2015;5:73974–9. https://doi.org/10.1039/C5RA14247D.
Zhu A, Bai S, Li L, Wang M, Wang J. Choline hydroxide: an efficient and biocompatible basic catalyst for the synthesis of biscoumarins under mild conditions. Catal Lett. 2015;145:1089–93. https://doi.org/10.1007/s10562-015-1487-6.
ParvanakBoroujeni K, Ghasemi P. Synthesis and application of a novel strong and stable supported ionic liquid catalyst with both Lewis and Brønsted acid sites. Catal Commun. 2013;37:50–4. https://doi.org/10.1016/j.catcom.2013.03.025.
Yang C, Su W-Q, Xu D-Z. Ionic liquid [Dabco-H][AcO] as a highly efficient and recyclable catalyst for the synthesis of various bisenol derivatives via domino Knoevenagel–Michael reaction in aqueous media. RSC Adv. 2016;6:99656–63. https://doi.org/10.1039/C6RA23018K.
Abbasi F, Azizi N, Abdoli-Senejani M. Highly efficient synthesis of dicoumarols and xanthene derivatives in presence of Brønsted–Lewis acidic ionic liquids catalyst. J Iran Chem Soc. 2017;14:2097–103. https://doi.org/10.1007/s13738-017-1146-5.
Singh P, Kumar P, Katyal A, Kalra R, Dass SK, Prakash S, Chandra R. Phosphotungstic acid: an efficient catalyst for the aqueous phase synthesis of bis-(4-hydroxycoumarin-3-yl)methanes. Catal Lett. 2010;134:303–8. https://doi.org/10.1007/s10562-009-0239-x.
Khurana JM, Kumar S. Tetrabutylammonium bromide (TBAB): a neutral and efficient catalyst for the synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives in water and solvent-free conditions. Tetrahedron Lett. 2009;50:4125–7. https://doi.org/10.1016/j.tetlet.2009.04.125.
Mehrabi H, Abusaidi H. Synthesis of biscoumarin and 3,4-dihydropyrano[c]chromene derivatives catalysed by sodium dodecyl sulfate (SDS) in neat water. J Iran Chem Soc. 2010;7:890–4. https://doi.org/10.1007/BF03246084.
Rezaei R, Moezzi F, Doroodmand MM. Propane-1,2,3-triyl tris(hydrogen sulfate): a mild and efficient recyclable catalyst for the synthesis of biscoumarin derivatives in water and solvent-free conditions. Chin Chem Lett. 2014;25:183–6. https://doi.org/10.1016/j.cclet.2013.10.033.
Davoodnia A. A highly efficient and fast method for the synthesis of biscoumarins using tetrabutylammonium hexatungstate [TBA]2 [W6O19] as green and reusable heterogeneous catalyst. Bull Korean Chem Soc. 2011;32:4286–90. https://doi.org/10.5012/bkcs.2011.32.12.4286.
Ghosh S, Mondal P, Das D, Tuhina K, Islam SKM. Use of PS-Zn-anthra complex as an efficient heterogeneous recyclable catalyst for carbon dioxide fixation reaction at atmospheric pressure and synthesis of dicoumarols under greener pathway. J Organomet Chem. 2018;866:1–12. https://doi.org/10.1016/j.jorganchem.2018.03.039.
Heravi MM, Nahavandi F, Sadjadi S, Oskooie HA, Bamoharram FF. Efficient synthesis of bis-coumarins using silica-supported preyssler nanoparticles. Synth Commun. 2010;40:498–503. https://doi.org/10.1080/00397910902985556.
Niknam K, Jamali A. Silica-bonded N-propylpiperazine sodium n-propionate as recyclable basic catalyst for synthesis of 3,4-dihydropyrano[c]chromene derivatives and biscoumarins. Chin J Catal. 2012;33:1840–9. https://doi.org/10.1016/S1872-2067(11)60457-9.
Sadeghi B, Ziya T. A fast, highly efficient, and green protocol for synthesis of biscoumarins catalyzed by silica sulfuric acid nanoparticles as a reusable catalyst. J Chem. 2013;2013:1–5. https://doi.org/10.1155/2013/179013.
Karimian R, Piri F, Safari AA, Davarpanah SJ. One-pot and chemoselective synthesis of bis(4-hydroxycoumarin) derivatives catalyzed by nano silica chloride. J Nanostruct Chem. 2013;3:52. https://doi.org/10.1186/2193-8865-3-52.
Ziarani GM, Badiei A, Azizi M, Lashgari N. Efficient one-pot synthesis of bis(4-hydroxycoumarin)methanes in the presence of sulfonic acid functionalized nanoporous silica (SBA-Pr-SO3H). J Chin Chem Soc. 2013;60:499–502. https://doi.org/10.1002/jccs.201200530.
Padalkar V, Phatangare K, Takale S, Pisal R, Chaskar A. Silica supported sodium hydrogen sulfate and indion 190 resin: an efficient and heterogeneous catalysts for facile synthesis of bis-(4-hydroxycoumarin-3-yl) methanes. J Saudi Chem Soc. 2015;19:42–5. https://doi.org/10.1016/j.jscs.2011.12.015.
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–2. https://doi.org/10.1038/359710a0.
Heravi MM, Daraie M. Mn(Pbdo)2Cl2/MCM-41 as a green catalyst in multi-component syntheses of some heterocycles. Res Chem Intermed. 2016;42:2979–88. https://doi.org/10.1007/s11164-015-2191-2.
Safaei-Ghomi J, Eshteghal F, Ghasemzadeh MA. Solvent-free synthesis of dihydropyrano[3,2-c]chromene and biscoumarin derivatives using magnesium oxide nanoparticles as a recyclable catalyst. Acta Chim Slov. 2014;61:703–8.
Shirini F, Fallah-Shojaei A, Samavi L, Abedini M. A clean synthesis of bis(indolyl)methane and biscoumarin derivatives using P4 VPy–CuO nanoparticles as a new, efficient and heterogeneous polymeric catalyst. RSC Adv. 2016;6:48469–78. https://doi.org/10.1039/C6RA04893E.
Shirini F, Lati MP. BiVO4-NPs: an efficient nano-catalyst for the synthesis of biscoumarins, bis(indolyl)methanes and 3,4-dihydropyrimidin-2(1H)-ones (thiones) derivatives. J Iran Chem Soc. 2017;14:75–87. https://doi.org/10.1007/s13738-016-0959-y.
Khodabakhshi S, Karami B, Eskandari K, Hoseini SJ, Nasrabadi H. Convenient on water synthesis of novel derivatives of dicoumarol as functional vitamin K depleter by Fe3O4 magnetic nanoparticles. Arab J Chem. 2017;10:S3907–12. https://doi.org/10.1016/j.arabjc.2014.05.030.
Hassanloie N, NorooziPesyan N, Sheykhaghaei G. Anchored Ni-dimethylglyoxime complex on Fe3O4@SiO2 core/shell nanoparticles for the clean catalytical synthesis of dicoumarols. Appl Organomet Chem. 2020. https://doi.org/10.1002/aoc.5242.
Khaskel A, Barman P, Jana U. Tyrosine loaded nanoparticles: an efficient catalyst for the synthesis of dicoumarols and hantzsch 1,4-dihydropyridines. RSC Adv. 2015;5:13366–73. https://doi.org/10.1039/C4RA16627B.
Fu Y, Lu Z, Fang K, He X, Huang H, Hu Y. Promiscuous enzyme-catalyzed cascade reaction in water: synthesis of dicoumarol derivatives. Bioorg Med Chem Lett. 2019;29:1236–40. https://doi.org/10.1016/j.bmcl.2019.03.007.
Timson D. Dicoumarol: a drug which hits at least two very different targets in vitamin K metabolism. Curr Drug Targets. 2017;18:500–10. https://doi.org/10.2174/1389450116666150722141906.
Harvison PJ. Dicumarol. In: xPharm: the comprehensive pharmacology reference. Amsterdam: Elsevier; 2007. p. 1–4.
2—Principles of herbal pharmacology. In: Bone K, Mills S, editors. Principles and practice of phytotherapy, 2nd edition. Churchill Livingstone: Saint Louis; 2013. p. 17–82. ISBN 978-0-443-06992-5.
Liu YT, Gong PH, Xiao FQ, Shao S, Zhao DQ, Yan MM, Yang XW. Chemical constituents and antioxidant, anti-inflammatory and anti-tumor activities of Melilotus officinalis (Linn.) Pall. Molecules. 2018. https://doi.org/10.3390/molecules23020271.
Keller C, Matzdorff A, Kemkes-Matthes B. Pharmacology of warfarin and clinical implications. Semin Thromb Hemost. 1999;25:13–6. https://doi.org/10.1055/s-2007-996418.
Dholariya HR, Patel KS, Patel JC, Patel KD. Dicoumarol complexes of Cu(II) based on 1,10-phenanthroline: synthesis, X-ray diffraction studies, thermal behavior and biological evaluation. Spectrochim Acta A Mol Biomol Spectrosc. 2013;108:319–28. https://doi.org/10.1016/j.saa.2012.09.096.
Han X, Chen C, Yan Q, Jia L, Taj A, Ma Y. Action of dicumarol on glucosamine-1-phosphate acetyltransferase of GLMU and Mycobacterium tuberculosis. Front Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.01799.
Lata S, Ali A, Sood V, Raja R, Banerjea AC. HIV-1 rev downregulates tat expression and viral replication via modulation of NAD(P)H: quinine oxidoreductase 1 (NQO1). Nat Commun. 2015;6:7244. https://doi.org/10.1038/ncomms8244.
Kammari K, Devaraya K, Bommakanti A, Kondapi AK. Development of pyridine dicoumarols as potent anti HIV-1 leads, targeting HIV-1 associated topoisomeraseIIβ kinase. Future Med Chem. 2017;9:1597–609. https://doi.org/10.4155/fmc-2017-0091.
Li J, Hou Z, Chen G-H, Li F, Zhou Y, Xue X-Y, Li Z-P, Jia M, Zhang Z-D, Li M-K, et al. Synthesis, antibacterial activities, and theoretical studies of dicoumarols. Org Biomol Chem. 2014;12:5528–35. https://doi.org/10.1039/C4OB00772G.
González-Aragón D, Ariza J, Villalba JM. Dicoumarol impairs mitochondrial electron transport and pyrimidine biosynthesis in human myeloid leukemia HL-60 cells. Biochem Pharmacol. 2007;73:427–39. https://doi.org/10.1016/j.bcp.2006.10.016.
Bello RI, Gómez-Díaz C, López-Lluch G, Forthoffer N, Córdoba-Pedregosa MC, Navas P, Villalba JM. Dicoumarol relieves serum withdrawal-induced G0/1 blockade in HL-60 cells through a superoxide-dependent mechanism. Biochem Pharmacol. 2005;69:1613–25. https://doi.org/10.1016/j.bcp.2005.03.012.
Du J, Daniels DH, Asbury C, Venkataraman S, Liu J, Spitz DR, Oberley LW, Cullen JJ. Mitochondrial production of reactive oxygen species mediate dicumarol-induced cytotoxicity in cancer cells. J Biol Chem. 2006;281:37416–26. https://doi.org/10.1074/jbc.M605063200.
Nolan KA, Zhao H, Faulder PF, Frenkel AD, Timson DJ, Siegel D, Ross D, Burke TR, Stratford IJ, Bryce RA. Coumarin-based inhibitors of human NAD(P)H: quinone oxidoreductase-1. identification, structure-activity, off-target effects and in vitro human pancreatic cancer toxicity. J Med Chem. 2007;50:6316–25. https://doi.org/10.1021/jm070472p.
Rehman S, Ikram M, Khan A, Min S, Azad E, Hofer TS, Mok KH, Baker RJ, Blake AJ, Rehman SU. New dicoumarol sodium compound: crystal structure, theoretical study and tumoricidal activity against osteoblast cancer cells. Chem Cent J. 2013;7:110. https://doi.org/10.1186/1752-153X-7-110.
Zhao XZ, Wu X-H. A small compound spindlactone A sensitizes human endometrial cancer cells to TRAIL-induced apoptosis via the inhibition of NAD(P)H dehydrogenase quinone I. Onco Targets Ther. 2018;11:3609–17. https://doi.org/10.2147/OTT.S165723.
Lewis A, Ough M, Li L, Hinkhouse MM, Ritchie JM, Spitz DR, Cullen JJ. Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin Cancer Res. 2004;10:4550–8. https://doi.org/10.1158/1078-0432.CCR-03-0667.
Wang L, Hu T, Shen J, Zhang L, Li L, Chan RL-Y, Li M, Wu WK-K, Cho C-H. Miltirone induced mitochondrial dysfunction and ROS-dependent apoptosis in colon cancer cells. Life Sci. 2016;151:224–34. https://doi.org/10.1016/j.lfs.2016.02.083.
Watanabe J, Nishiyama H, Matsui Y, Ito M, Kawanishi H, Kamoto T, Ogawa O. Dicoumarol potentiates cisplatin-induced apoptosis mediated by c-Jun N-terminal kinase in P53 wild-type urogenital cancer cell lines. Oncogene. 2006;25:2500–8. https://doi.org/10.1038/sj.onc.1209162.
Matsui Y, Watanabe J, Ding S, Nishizawa K, Kajita Y, Ichioka K, Saito R, Kobayashi T, Ogawa O, Nishiyama H. Dicoumarol enhances doxorubicin-induced cytotoxicity in P53 wild-type urothelial cancer cells through P38 activation. BJU Int. 2010;105:558–64. https://doi.org/10.1111/j.1464-410X.2009.08732.x.
He D, Gu F, Wu J, Gu X-T, Lu C-X, Mao A, Zhang G, Ding Z, Wang J, Hao J, et al. Targeting PSG1 to enhance chemotherapeutic efficacy: new application for anti-coagulant the dicumarol. Clin Sci. 2016;130:2267–76. https://doi.org/10.1042/CS20160536.
Park EJ, Min K, Choi KS, Kwon TK. Dicoumarol sensitizes renal cell carcinoma Caki cells to TRAIL-induced apoptosis through down-regulation of Bcl-2, Mcl-1 and c-FLIP in a NQO1-independent manner. Exp Cell Res. 2014;323:144–54. https://doi.org/10.1016/j.yexcr.2014.01.009.
Zhang W, Su J, Xu H, Yu S, Liu Y, Zhang Y, Sun L, Yue Y, Zhou X. Dicumarol inhibits PDK1 and targets multiple malignant behaviors of ovarian cancer cells. PLoS ONE. 2017;12: e0179672. https://doi.org/10.1371/journal.pone.0179672.
Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu J, Zhang YP, Weydert CJD, Domann FE, Oberley LW. Dicumarol inhibition of NADPH: quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003;63:5513–20.
Soldani C, Scovassi AI. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–8. https://doi.org/10.1023/A:1016119328968.
Buranrat B, Prawan A, Kukongviriyapan U, Kongpetch S, Kukongviriyapan V. Dicoumarol enhances gemcitabine-induced cytotoxicity in high NQO1-expressing cholangiocarcinoma cells. World J Gastroenterol. 2010;16:2362–70. https://doi.org/10.3748/wjg.v16.i19.2362.
Hernández A, López-Lluch G, Bernal JA, Navas P, Pintor-Toro JA. Dicoumarol down-regulates human PTTG1/securin MRNA expression through inhibition of Hsp90. Mol Cancer Ther. 2008;7:474–82. https://doi.org/10.1158/1535-7163.MCT-07-0457.
Jones W, Bianchi K. Aerobic glycolysis: beyond proliferation. Front Immunol. 2015;6:227. https://doi.org/10.3389/fimmu.2015.00227.
Funding
This work was supported by LAQV (UIDB/50006/2020), CIAFEL (UID/DTP/00617/2020), ITR (LA/P/0064/2020) and CITAB (UIDB/04033/2020) research units and by A.M.P.’s (SFRH/BD/144396/2019), by R.S.R’s (2022.14518.BD), and T.F.’s fellowship (2020.04789.BD) through national founds by the Portuguese Foundation for Science and Technology (FCT) and co-financed by the European Regional Development Fund (FEDER), within the PT2020 Partnership Agreement.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is, revising or critically reviewing the article; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Silva, V.L.M., Silva-Reis, R., Moreira-Pais, A. et al. Dicoumarol: from chemistry to antitumor benefits. Chin Med 17, 145 (2022). https://doi.org/10.1186/s13020-022-00699-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-022-00699-0












